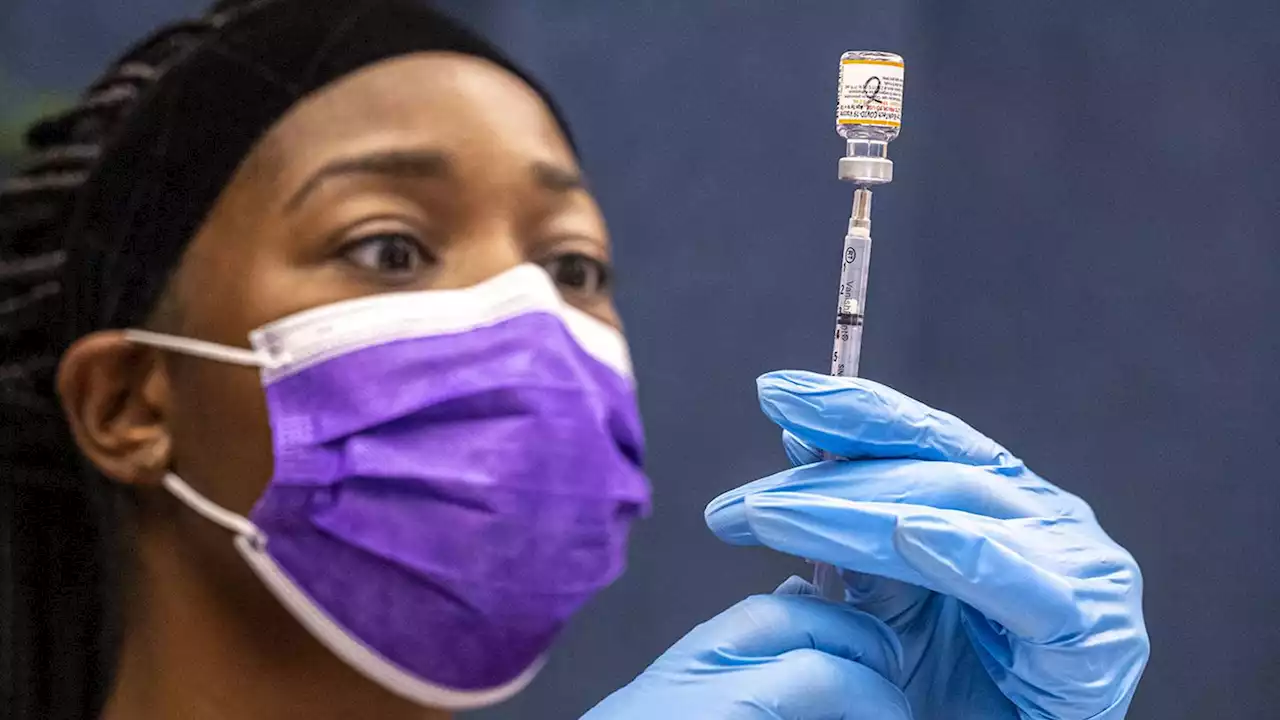
The reformulated COVID-19 shots are now available for children as young as 6 months old.
Officials said that as of Thursday, children between the ages of 6 months and 5 years who were vaccinated with the original Moderna shots are eligible to get a booster of the updated Moderna shot two months after completing a primary series of the original formulation.
Children from 6 months to 4 years old who have not yet begun getting original vaccines developed by Pfizer-BioNTech or who have gotten only their first two of three doses will be eligible to get the newly authorized shots. Officials said that children in that age group who have already gotten their three-dose primary series of Pfizer-BioNTech shots will not be eligible for the updated vaccines.
As of last week, nearly 1.8 million Americans under the age of 5 have gotten at least one dose of any of the available COVID-19 vaccines,In October, officials recommended the bivalent vaccines for children as young as 5.More than 80% of the total U.S. population has gotten at least one COVID-19 vaccine shot, according to the CDC.
Since the start of the pandemic, officials have confirmed more than 99.3 million COVID-19 infections and reported over 1 million deaths nationwide, . More than 647.4 million COVID-19 cases have been reported worldwide, resulting in 6.6 million deaths, according to the university.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 COVID-19 cases Illinois: IL reports 4,019 new coronavirus cases, 9 new deathsIllinois reported 4,019 new COVID cases and 9 new deaths Wednesday. Multiple Chicago area counites have been raised to a 'medium' risk level.
COVID-19 cases Illinois: IL reports 4,019 new coronavirus cases, 9 new deathsIllinois reported 4,019 new COVID cases and 9 new deaths Wednesday. Multiple Chicago area counites have been raised to a 'medium' risk level.
Read more »
 Illinois Coronavirus Updates: Metrics Rising, Duckworth Tests Positive for COVIDHere’s what you need to know about the coronavirus pandemic across Illinois today.
Illinois Coronavirus Updates: Metrics Rising, Duckworth Tests Positive for COVIDHere’s what you need to know about the coronavirus pandemic across Illinois today.
Read more »
 COVID-19 cases Illinois: IL reports 3,334 new coronavirus cases, 9 new deathsIllinois reported 3,334 new COVID cases and 9 new deaths Thursday. Multiple Chicago area counites have been raised to a 'medium' risk level.
COVID-19 cases Illinois: IL reports 3,334 new coronavirus cases, 9 new deathsIllinois reported 3,334 new COVID cases and 9 new deaths Thursday. Multiple Chicago area counites have been raised to a 'medium' risk level.
Read more »
 FDA gives nod to updated coronavirus shots for young childrenThe youngest Americans will have access to variant-targeting boosters already available to older children and adults.
FDA gives nod to updated coronavirus shots for young childrenThe youngest Americans will have access to variant-targeting boosters already available to older children and adults.
Read more »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Read more »
 U.S. FDA authorizes bivalent COVID shots for kids as young as 6 months oldThe amended authorization on Thursday from the Food and Drug Administration allows use of Moderna's bivalent shot as a booster in children 6 months through 5 years of age, two months after their initial vaccination. Pfizer/BioNTech's updated shot can now be given as a third dose to those aged 6 months through 4 years, who have not completed their primary vaccination series or are yet to receive the third dose. Children who have completed their initial three-dose vaccination with Pfizer's original shot are not yet eligible to receive the bivalent booster, the agency said.
U.S. FDA authorizes bivalent COVID shots for kids as young as 6 months oldThe amended authorization on Thursday from the Food and Drug Administration allows use of Moderna's bivalent shot as a booster in children 6 months through 5 years of age, two months after their initial vaccination. Pfizer/BioNTech's updated shot can now be given as a third dose to those aged 6 months through 4 years, who have not completed their primary vaccination series or are yet to receive the third dose. Children who have completed their initial three-dose vaccination with Pfizer's original shot are not yet eligible to receive the bivalent booster, the agency said.
Read more »