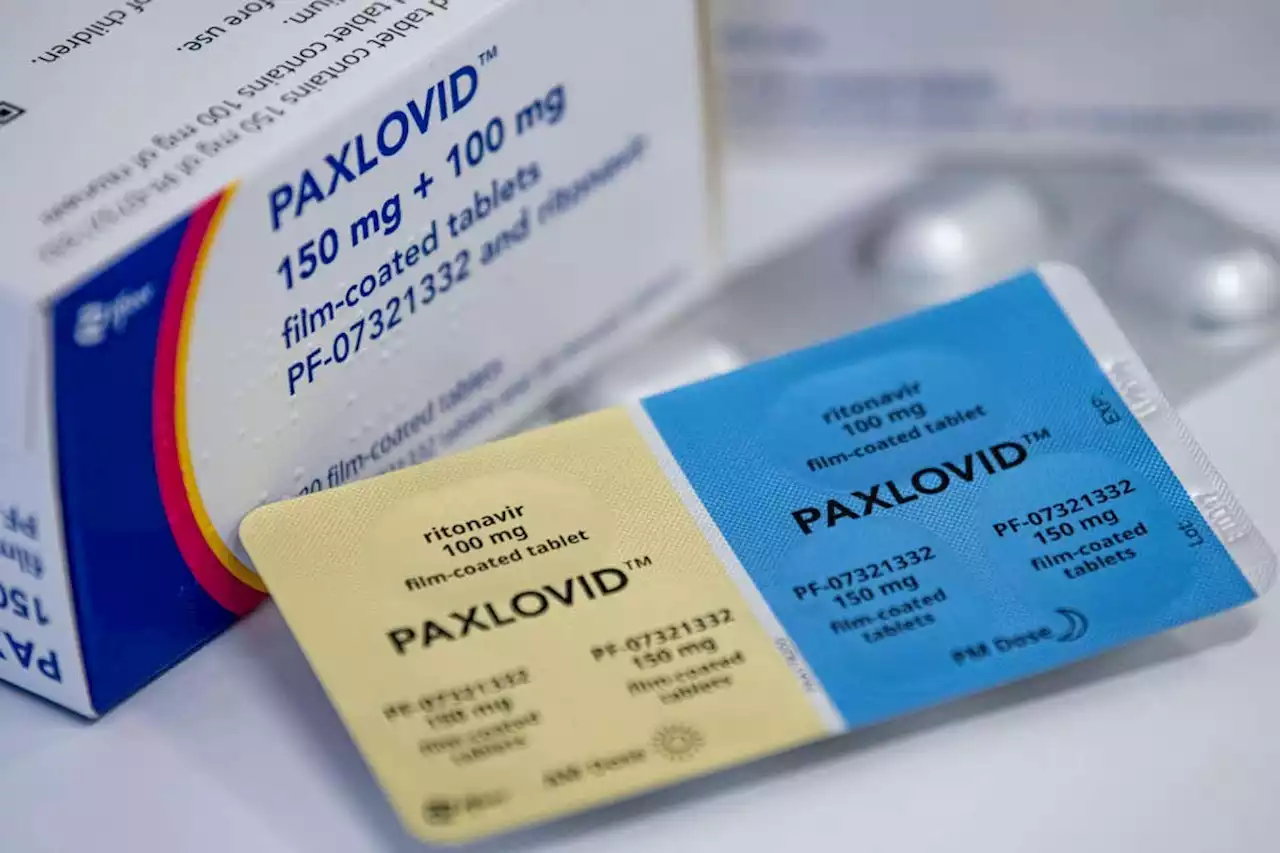
In the past few weeks, some people are reported to have experienced viral “rebound” after coming off Paxlovid, a drug that prevents severe covid-19 in vulnerable people.
Fabian Sommer/dpa/Alamymay not eradicate the virus in everyone. Several countries – including the US, UK, Canada, Israel and France – may prescribe a five-day course of the Pfizer drug Paxlovid, which can be taken at home, to people who are particularly vulnerable to covid-19’s complications.
In the trial that led to its approval, Paxlovid cut the risk of a covid-19-related hospitalisation or death by 88 per cent. But emerging reports now suggest …App + Web
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
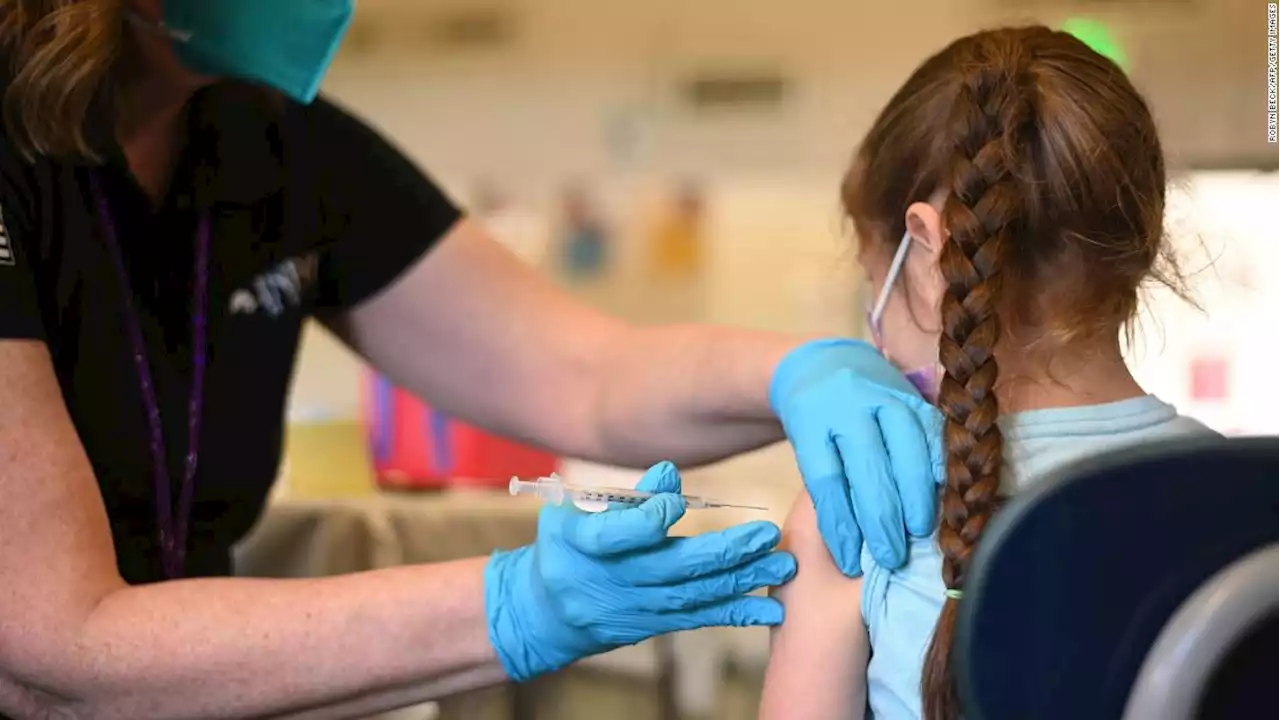 FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
Read more »
 FDA authorizes Pfizer COVID-19 booster for kids 5-11A rise in new COVID-19 cases among children was reported overnight by health leaders but there may be extra protection coming for those children in the form of booster doses of vaccines. Andrea Nakano reports. (5-17-22)
FDA authorizes Pfizer COVID-19 booster for kids 5-11A rise in new COVID-19 cases among children was reported overnight by health leaders but there may be extra protection coming for those children in the form of booster doses of vaccines. Andrea Nakano reports. (5-17-22)
Read more »