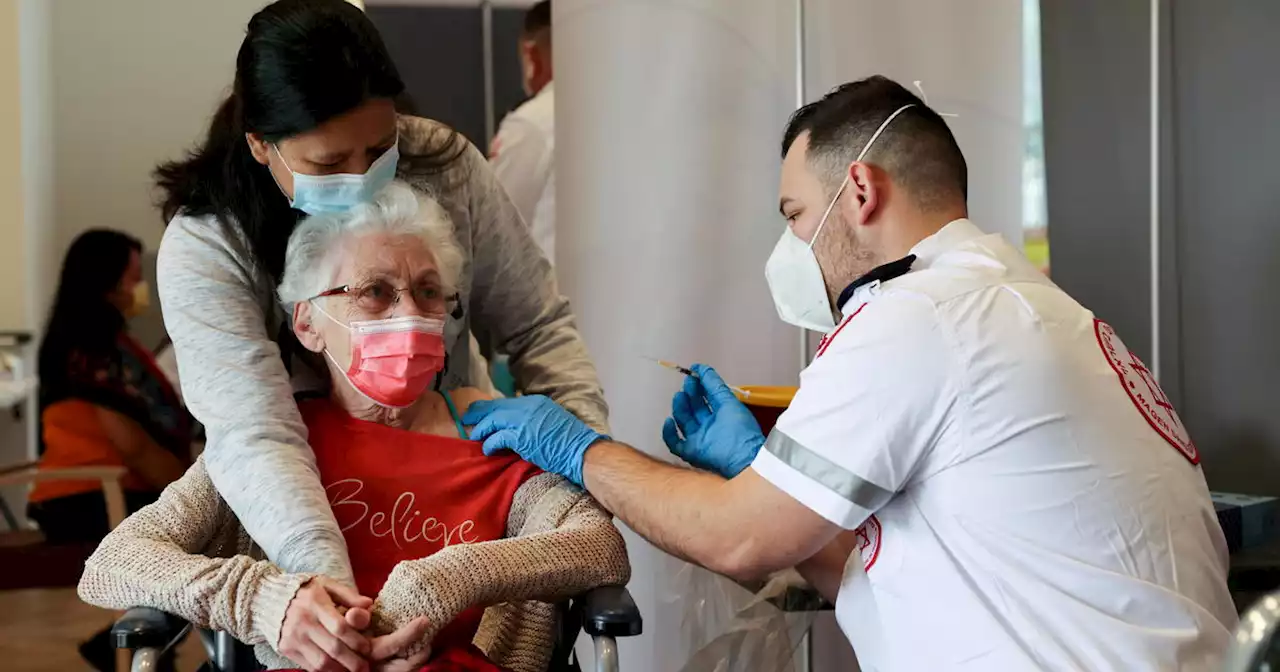
FDA authorizes second booster of COVID vaccine for ages 50 and over
Tuesday it had authorized a new round of the Moderna or Pfizer and BioNTech COVID-19 vaccines for those who want them. signing off
"We're hoping that by taking this action, we will help allow people to take steps to protect themselves should we have another wave that comes through this country," the FDA's top vaccines official, Dr. Peter Marks,Americans who are old enough to be eligible will be able to get the second booster as early as four months after their first booster. Immunocompromised Americans ages 12 and older who were boosted will also be able to get another shot.
Both Moderna as well as Pfizer and BioNTech are also pursuing new versions of their vaccines that could be rolled out later this year. The companies hope the revised shots will outperform current formulations that were designed to target the initial"prototype virus."
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
Read more »
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
 FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Read more »
 FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
Read more »