The decision comes as cases start to tick up again.
The Food and Drug Administration authorized a second booster dose of the Pfizer / BioNTech and Moderna COVID-19 vaccines for people over 50 or who are immunocompromised, the agencyThe decision comes as the United States heads into yet another wave of infections, this time driven by an even more transmissible version of the omicron variant of the virus.
The FDA said in aOnce again, the FDA isas the foundation for its decision-making around COVID-19 vaccines — in this case, data from Israel, which found a lower rate of serious illness and death in older adults who had two boosters compared with just one.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Read more »
 Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Read more »
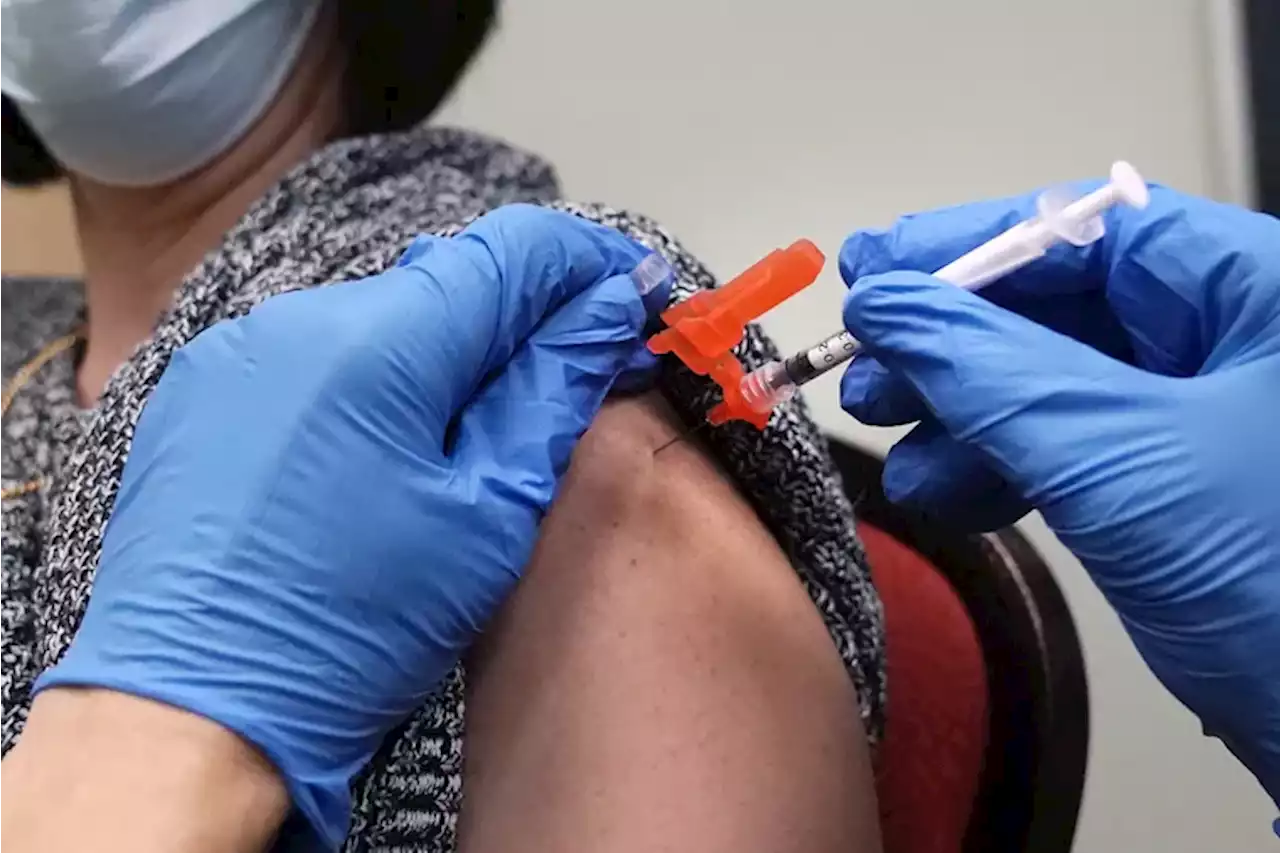 The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
Read more »
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
