Breaking: The FDA inappropriately collaborated with Biogen before approving its Alzheimer's drug Aduhelm, a report by House Democrats found
Biogen’s Aduhelm is the first approved treatment for early-stage Alzheimer’s patients that might be able to slow the disease. WSJ explains how the drug interacts with brain cells, and why some doctors aren’t ready to prescribe it. Illustration: Jacob ReynoldsThe U.S. Food and Drug Administration “inappropriately” collaborated with Biogen Inc.
Biogen internal documents obtained through a congressional investigation also showed that the company expected pushback from patients and payers but priced its drug at $56,000 to maximize profit, the report says.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Kala Pharmaceuticals stock rockets after FDA accepts IND application for PCED treatmentShares of Kala Pharmaceuticals Inc. shot up 45.7% in premarket trading Wednesday, after the biopharmaceutical company said the Food and Drug Administration...
Kala Pharmaceuticals stock rockets after FDA accepts IND application for PCED treatmentShares of Kala Pharmaceuticals Inc. shot up 45.7% in premarket trading Wednesday, after the biopharmaceutical company said the Food and Drug Administration...
Read more »
 FDA Fast-Tracks Approval of Overdose Reversal Drug for OTC UseAn inexpensive overdose-reversal drug in nasal spray form is being fast-tracked by the FDA for consideration to be sold over-the-counter.
FDA Fast-Tracks Approval of Overdose Reversal Drug for OTC UseAn inexpensive overdose-reversal drug in nasal spray form is being fast-tracked by the FDA for consideration to be sold over-the-counter.
Read more »
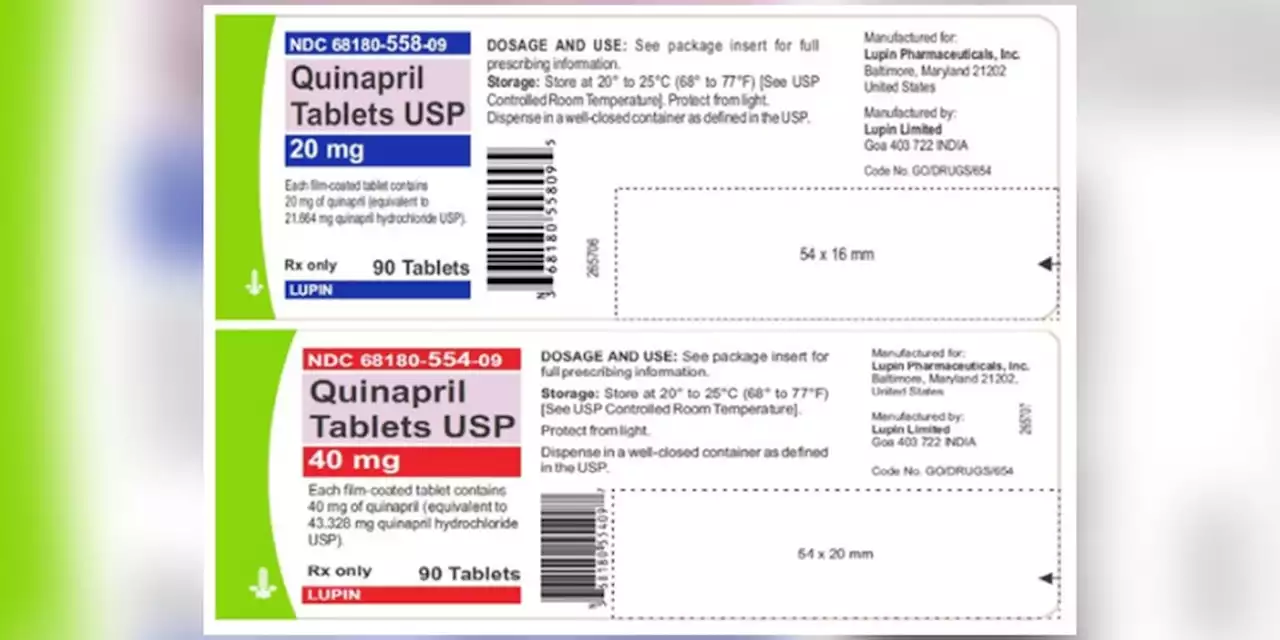 Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Read more »
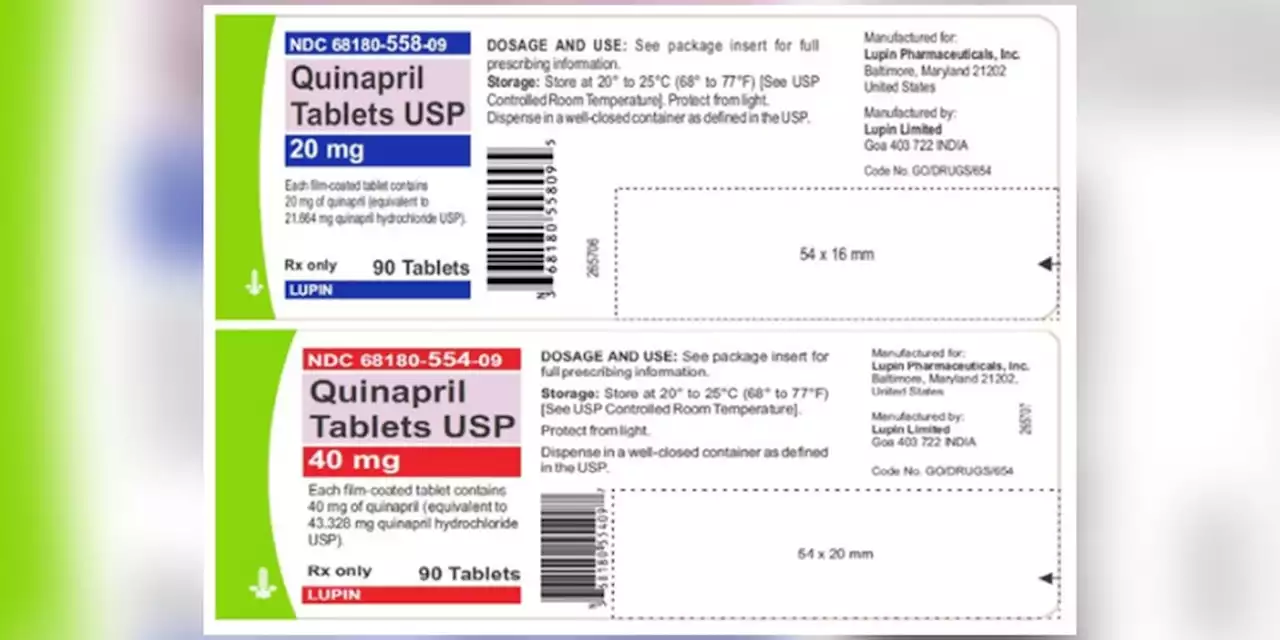 Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Read more »
 Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Read more »
 Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Read more »
