Column: How our cells strategize to keep up alive
Wait, what? Is that even possible? Apparently it is. Over half of you is water, and we know that we constantly replace that. Another large percentage of you is protein, and as you may recall,
. We even disassemble and replace our ribosomes and large organelles such as mitochondria, which are made primarily of protein.constantly replacing their seemingly permanent structures and old battered molecular machines with new ones. The only ones they don’t replace are our massive chromosomes. Instead, we have machines that swarm along them looking for problems and fixing them.
What if the damage to a cell is too great to repair? We have a fallback plan for that too. We simply destroy the entire cell, chop it up into recyclable units, and make a fresh one. On average, you replace most of your cells, which amounts to about 330 billion cells a day. Those that work in the harshest conditions are retired most frequently. The damage to many cells in your intestines, which are exposed to harsh acids, is so predictable that they commit planned suicide and are replaced.
So, in addition to using reliable machines, our cells have a three-pronged motto to stay alive: ceaselessly check for errors, constantly repair, and continually replace. In a way, your body is like a major New York highway—always open and always under repair.by Dan Levitt. To be published by HarperCollins on Jan. 24, 2023. Copyright © 2023 by Daniel Levitt. All rights reserved.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Frontiers | Isolation of a cytolytic subpopulation of extracellular vesicles derived from NK cells containing NKG7 and cytolytic proteinsNK cells can broadly target and kill malignant cells via release of cytolytic proteins. NK cells also release extracellular vesicles (EVs) that contain cytolytic proteins, previously shown to induce apoptosis of a variety of cancer cells in vitro and in vivo. The EVs released by NK cells are likely very heterogeneous, as vesicles can be released from the plasma membrane or from different intracellular compartments. In this study, we undertook a fractionation scheme to enrich for cytolytic NK-EVs. NK-EVs were harvested from culture medium from the human NK-92 cell line or primary human NK cells grown in serum-free conditions. By combining ultracentrifugation with downstream density-gradient ultracentrifugation or size-exclusion chromatography, distinct EV populations were identified. Density-gradient ultracentrifugation led to separation of three subpopulations of EVs. The different EV isolates were characterized by label-free quantitative mass spectrometry and western blotting, and we found that one subpopulation was primarily enriched for plasma membrane proteins and tetraspanins CD37, CD82, and CD151, and likely represents microvesicles. The other major subpopulation was enriched in intracellularly derived markers with high expression of the endosomal tetraspanin CD63 and markers for intracellular organelles. The intracellularly derived EVs were highly enriched in cytolytic proteins, and possessed high apoptotic activity against HCT-116 colon cancer spheroids. To further enrich for cytolytic EVs, immunoaffinity pulldowns led to the isolation of a subset of EVs containing the cytolytic granule marker NKG7 and the majority of vesicular granzyme B content. We therefore propose that EVs containing cytolytic proteins may primarily be released via cytolytic granules.
Frontiers | Isolation of a cytolytic subpopulation of extracellular vesicles derived from NK cells containing NKG7 and cytolytic proteinsNK cells can broadly target and kill malignant cells via release of cytolytic proteins. NK cells also release extracellular vesicles (EVs) that contain cytolytic proteins, previously shown to induce apoptosis of a variety of cancer cells in vitro and in vivo. The EVs released by NK cells are likely very heterogeneous, as vesicles can be released from the plasma membrane or from different intracellular compartments. In this study, we undertook a fractionation scheme to enrich for cytolytic NK-EVs. NK-EVs were harvested from culture medium from the human NK-92 cell line or primary human NK cells grown in serum-free conditions. By combining ultracentrifugation with downstream density-gradient ultracentrifugation or size-exclusion chromatography, distinct EV populations were identified. Density-gradient ultracentrifugation led to separation of three subpopulations of EVs. The different EV isolates were characterized by label-free quantitative mass spectrometry and western blotting, and we found that one subpopulation was primarily enriched for plasma membrane proteins and tetraspanins CD37, CD82, and CD151, and likely represents microvesicles. The other major subpopulation was enriched in intracellularly derived markers with high expression of the endosomal tetraspanin CD63 and markers for intracellular organelles. The intracellularly derived EVs were highly enriched in cytolytic proteins, and possessed high apoptotic activity against HCT-116 colon cancer spheroids. To further enrich for cytolytic EVs, immunoaffinity pulldowns led to the isolation of a subset of EVs containing the cytolytic granule marker NKG7 and the majority of vesicular granzyme B content. We therefore propose that EVs containing cytolytic proteins may primarily be released via cytolytic granules.
Read more »
 Dead Cells' Return to Castlevania DLC gets first, brief gameplay trailerDecember brought the surprise news that Dead Cells - the superb rogue-like action-platformer from developers Motion Twi…
Dead Cells' Return to Castlevania DLC gets first, brief gameplay trailerDecember brought the surprise news that Dead Cells - the superb rogue-like action-platformer from developers Motion Twi…
Read more »
 Frontiers | Prolyl-hydroxylase inhibitor-induced regeneration of alveolar bone and soft tissue in a mouse model of periodontitis through metabolic reprogrammingBone injuries and fractures reliably heal through a process of regeneration with restoration to original structure and function when the gap between adjacent sides of a fracture site is small. However, when there is significant volumetric loss of bone, bone regeneration usually does not occur. In the present studies, we explore a particular case of volumetric bone loss in a mouse model of human periodontal disease (PD) in which alveolar bone surrounding teeth is permanently lost and not replaced. This model employs the placement of a ligature around the upper second molar for 10 days leading to inflammation and bone breakdown and closely replicates the bacterially-induced inflammatory etiology of human PD to induce bone degeneration. After ligature removal, mice are treated with a timed-release formulation of a small molecule inhibitor of prolylhydroxylases (PHDi; 1,4-DPCA) previously shown to induce epimorphic regeneration of soft tissue in non-regenerating mice. This PHDi induces high expression of HIF-1α and is able to shift the metabolic state from OXPHOS to aerobic glycolysis, an energetic state used by stem cells and embryonic tissue. This regenerative response was completely blocked by siHIF1a. In these studies, we show that timed-release 1,4-DPCA rapidly and completely restores PD-affected bone and soft tissue with normal anatomic fidelity and with increased stem cell markers due to site-specific stem cell migration and/or de-differentiation of local tissue, periodontal ligament (PDL) cell proliferation, and increased vascularization. In-vitro studies using gingival tissue show that 1,4-DPCA indeed induces de-differentiation and the expression of stem cell markers but does not exclude the role of migrating stem cells. Evidence of metabolic reprogramming is seen by the expression of not only HIF-1a, its gene targets, and resultant de-differentiation markers, but also the metabolic genes Glut-1, Gapdh, Pdk1, Pgk1 and Ldh-a in the periodontal tissue.
Frontiers | Prolyl-hydroxylase inhibitor-induced regeneration of alveolar bone and soft tissue in a mouse model of periodontitis through metabolic reprogrammingBone injuries and fractures reliably heal through a process of regeneration with restoration to original structure and function when the gap between adjacent sides of a fracture site is small. However, when there is significant volumetric loss of bone, bone regeneration usually does not occur. In the present studies, we explore a particular case of volumetric bone loss in a mouse model of human periodontal disease (PD) in which alveolar bone surrounding teeth is permanently lost and not replaced. This model employs the placement of a ligature around the upper second molar for 10 days leading to inflammation and bone breakdown and closely replicates the bacterially-induced inflammatory etiology of human PD to induce bone degeneration. After ligature removal, mice are treated with a timed-release formulation of a small molecule inhibitor of prolylhydroxylases (PHDi; 1,4-DPCA) previously shown to induce epimorphic regeneration of soft tissue in non-regenerating mice. This PHDi induces high expression of HIF-1α and is able to shift the metabolic state from OXPHOS to aerobic glycolysis, an energetic state used by stem cells and embryonic tissue. This regenerative response was completely blocked by siHIF1a. In these studies, we show that timed-release 1,4-DPCA rapidly and completely restores PD-affected bone and soft tissue with normal anatomic fidelity and with increased stem cell markers due to site-specific stem cell migration and/or de-differentiation of local tissue, periodontal ligament (PDL) cell proliferation, and increased vascularization. In-vitro studies using gingival tissue show that 1,4-DPCA indeed induces de-differentiation and the expression of stem cell markers but does not exclude the role of migrating stem cells. Evidence of metabolic reprogramming is seen by the expression of not only HIF-1a, its gene targets, and resultant de-differentiation markers, but also the metabolic genes Glut-1, Gapdh, Pdk1, Pgk1 and Ldh-a in the periodontal tissue.
Read more »
 Stopping a rare childhood cancer in its tracksScientists at Cold Spring Harbor Laboratory (CSHL) have discovered a new drug target for Ewing sarcoma, a rare kind of cancer usually diagnosed in children and young adults. Their experiments show that the cells causing this cancer can essentially be reprogrammed with the flick of a genetic switch.
Stopping a rare childhood cancer in its tracksScientists at Cold Spring Harbor Laboratory (CSHL) have discovered a new drug target for Ewing sarcoma, a rare kind of cancer usually diagnosed in children and young adults. Their experiments show that the cells causing this cancer can essentially be reprogrammed with the flick of a genetic switch.
Read more »
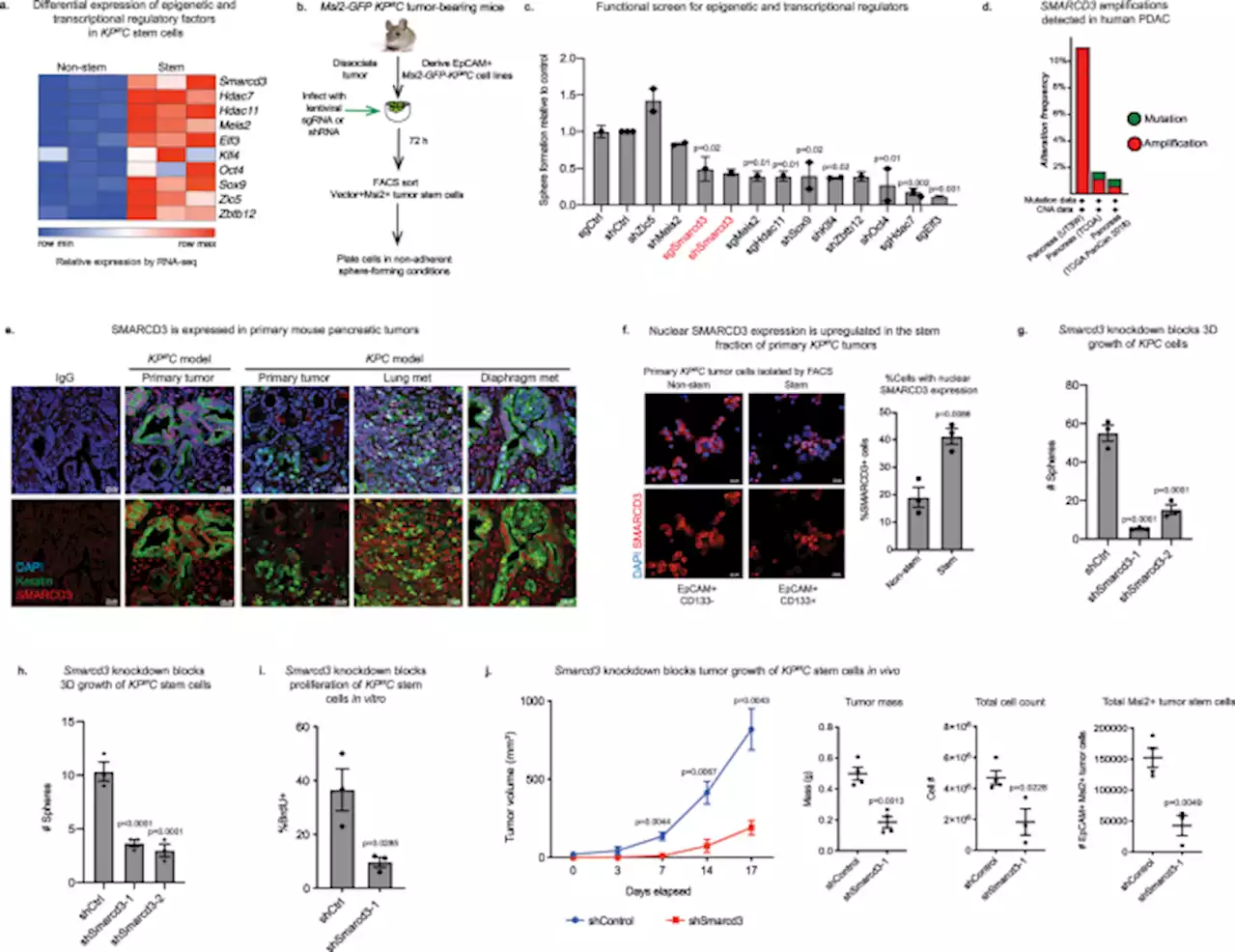 Smarcd3 is an epigenetic modulator of the metabolic landscape in pancreatic ductal adenocarcinoma - Nature CommunicationsClinical management of pancreatic cancer remains challenging. Here, the authors suggest SMARCD3 as a potential epigenetic dependency establishing the metabolic landscape in aggressive pancreatic cancer cells and as a potential therapeutic target in pancreatic cancer.
Smarcd3 is an epigenetic modulator of the metabolic landscape in pancreatic ductal adenocarcinoma - Nature CommunicationsClinical management of pancreatic cancer remains challenging. Here, the authors suggest SMARCD3 as a potential epigenetic dependency establishing the metabolic landscape in aggressive pancreatic cancer cells and as a potential therapeutic target in pancreatic cancer.
Read more »
