The first vaccine to protect older people against RSV could gain federal approval next spring, Pfizer announced.
the Food and Drug Administration has agreed to a priority review of its candidate vaccine for respiratory syncytial virus for people 60 and older.
RSV kills about 14,000 older Americans a year and sends about 100,000 people to the hospital. The common virus is highly contagious and usually causes mild cold-like symptoms but can be fatal for young children or older adults.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Expected to Decide on Pfizer RSV Vaccine for Older Adults by May 2023Pfizer said the FDA has accepted its RSV vaccine candidate for review under an expedited process that reduces the approval process by four months.
FDA Expected to Decide on Pfizer RSV Vaccine for Older Adults by May 2023Pfizer said the FDA has accepted its RSV vaccine candidate for review under an expedited process that reduces the approval process by four months.
Read more »
 What to know about Pfizer's RSV vaccine for older adults as FDA prepares to reviewAn RSV vaccine for older adults could be on the horizon after Pfizer announced the FDA would fast-track review of its experimental vaccine.
What to know about Pfizer's RSV vaccine for older adults as FDA prepares to reviewAn RSV vaccine for older adults could be on the horizon after Pfizer announced the FDA would fast-track review of its experimental vaccine.
Read more »
 FDA set to decide on Pfizer's RSV vaccine by MayPfizer Inc. undefined said Wednesday that its experimental respiratory syncytial virus vaccine received Priority Review from the Food and Drug...
FDA set to decide on Pfizer's RSV vaccine by MayPfizer Inc. undefined said Wednesday that its experimental respiratory syncytial virus vaccine received Priority Review from the Food and Drug...
Read more »
 U.S. FDA grants priority review to Pfizer's RSV vaccinePfizer Inc said on Wednesday the U.S. Food and Drug Administration will review its respiratory syncytial virus (RSV) vaccine candidate on priority.
U.S. FDA grants priority review to Pfizer's RSV vaccinePfizer Inc said on Wednesday the U.S. Food and Drug Administration will review its respiratory syncytial virus (RSV) vaccine candidate on priority.
Read more »
 Pfizer asks FDA to clear updated COVID-19 vaccine for kids under 5If the Food and Drug Administration agrees, a dose of Pfizer's bivalent omicron-targeting vaccine would be substituted for their third shot.
Pfizer asks FDA to clear updated COVID-19 vaccine for kids under 5If the Food and Drug Administration agrees, a dose of Pfizer's bivalent omicron-targeting vaccine would be substituted for their third shot.
Read more »
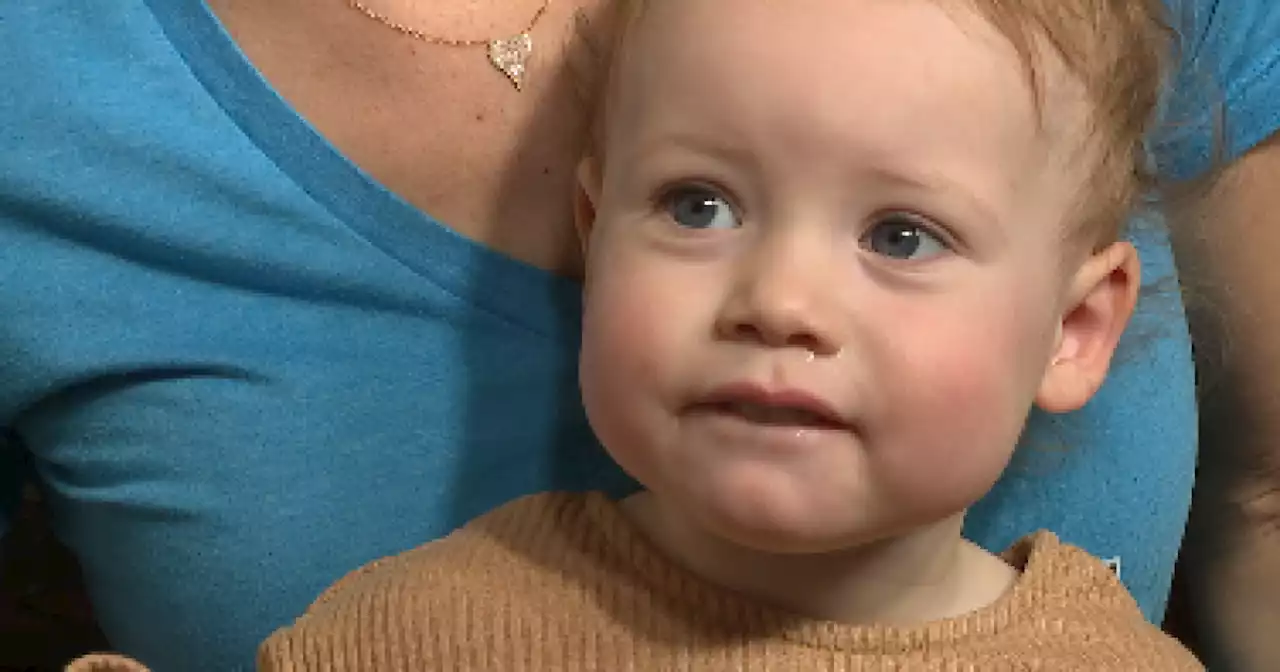 Parents of RSV survivor says they waited 24 hours for hospital bed to openAn 18-month-old was released from the hospital Sunday, after she survived the battle against RSV, spending four days in Summerlin Hospital. Her parents say it was their worst nightmare.
Parents of RSV survivor says they waited 24 hours for hospital bed to openAn 18-month-old was released from the hospital Sunday, after she survived the battle against RSV, spending four days in Summerlin Hospital. Her parents say it was their worst nightmare.
Read more »
