Researchers explore the correlation between the dynamic shedding pattern of SARS-CoV-2 and viral load natureportfolio UNIGEnews SARSCoV2 COVID19 ViralLoad SheddingPattern
By Neha MathurDec 7 2022Reviewed by Aimee Molineux In a recent article published in Nature Reviews Microbiology, researchers attempted to establish an association between severe acute respiratory syndrome coronavirus 2 viral load, indicating its ribonucleic acid levels, and the presence of infectious virions.
A detailed understanding of viral shedding patterns is crucial for designing public health interventions to contain human-to-human SARS-CoV-2 transmission. Although multifactorial, biological characteristics of SARS-CoV-2 and its variants, host factors, and pre-existing immunity of the diseased individual affect the shedding of its infectious virions, which, in turn, determines its onward transmission.
Contrastingly, RT-PCR estimates infectiousness qualitatively or quantitatively by viral replication in cell culture. Clinical samples with lower viral load often show delayed development of a cytopathic effect . Likewise, there are other methods for infectious virion quantification, such as plaque assays and focus-forming assays, to name a few.
Despite its high transmissibility, Omicron led to much lower RNA viral loads and cell culture isolation probability. Studies have observed different behaviors of Omicron sublineages concerning infectious virus titers, with Omicron BA.2 leading to higher RNA viral loads and taking more time to clear infection than Omicron BA.1.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
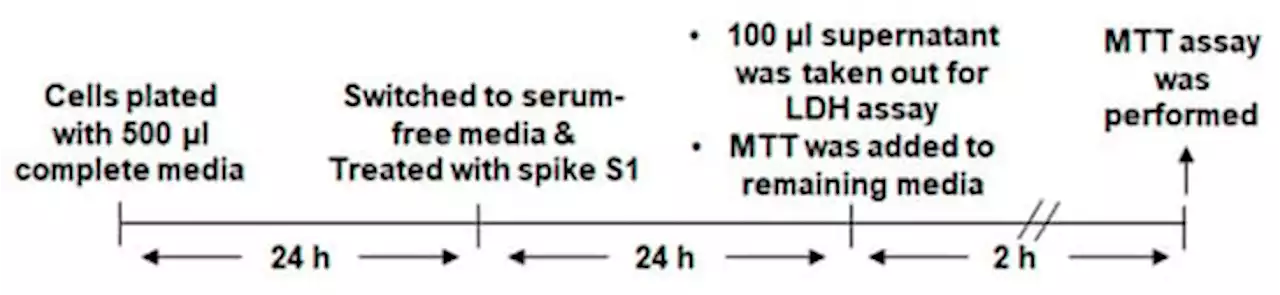 Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1This study underlines the importance of SARS-CoV-2 spike S1 in prompting death in cultured non-small cell lung cancer (NSCLC) cells and in vivo in lung tumors in mice. Interestingly, we found that recombinant spike S1 treatment at very low doses led to death of human A549 NSCLC cells. On the other hand, boiled recombinant SARS-CoV-2 spike S1 remained unable to induce death, suggesting that the induction of cell death in A549 cells was due to native SARS-CoV-2 spike S1 protein. SARS-CoV-2 spike S1-induced A549 cell death was also inhibited by neutralizing antibodies against spike S1 and ACE2. Moreover, our newly designed wild type ACE2-interacting domain of SARS-CoV-2 (wtAIDS), but not mAIDS, peptide also attenuated SARS-CoV-2 spike S1-induced cell death, suggesting that SARS-CoV-2 spike S1-induced death in A549 NSCLC cells depends on its interaction with ACE2 receptor. Similarly, recombinant spike S1 treatment also led to death of human H1299 and H358 NSCLC cells. Finally, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) intoxication led to the formation tumors in lungs of A/J mice and alternate day intranasal treatment with low dose of recombinant SARS-CoV-2 spike S1 from 22-weeks of NNK insult (late stage) induced apoptosis and tumor regression in the lungs. These studies indicate that SARS-CoV-2 spike S1 may have implications for lung cancer treatment.
Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1This study underlines the importance of SARS-CoV-2 spike S1 in prompting death in cultured non-small cell lung cancer (NSCLC) cells and in vivo in lung tumors in mice. Interestingly, we found that recombinant spike S1 treatment at very low doses led to death of human A549 NSCLC cells. On the other hand, boiled recombinant SARS-CoV-2 spike S1 remained unable to induce death, suggesting that the induction of cell death in A549 cells was due to native SARS-CoV-2 spike S1 protein. SARS-CoV-2 spike S1-induced A549 cell death was also inhibited by neutralizing antibodies against spike S1 and ACE2. Moreover, our newly designed wild type ACE2-interacting domain of SARS-CoV-2 (wtAIDS), but not mAIDS, peptide also attenuated SARS-CoV-2 spike S1-induced cell death, suggesting that SARS-CoV-2 spike S1-induced death in A549 NSCLC cells depends on its interaction with ACE2 receptor. Similarly, recombinant spike S1 treatment also led to death of human H1299 and H358 NSCLC cells. Finally, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) intoxication led to the formation tumors in lungs of A/J mice and alternate day intranasal treatment with low dose of recombinant SARS-CoV-2 spike S1 from 22-weeks of NNK insult (late stage) induced apoptosis and tumor regression in the lungs. These studies indicate that SARS-CoV-2 spike S1 may have implications for lung cancer treatment.
Read more »
 Co-infecting pathogens and the microbiome from SARS-CoV-2 positive and negative samplesCo-infecting pathogens and the microbiome from SARS-CoV-2 positive and negative samples PLOSONE jgi pathogen microbiome SARSCoV2 COVID19 coronavirus covid
Co-infecting pathogens and the microbiome from SARS-CoV-2 positive and negative samplesCo-infecting pathogens and the microbiome from SARS-CoV-2 positive and negative samples PLOSONE jgi pathogen microbiome SARSCoV2 COVID19 coronavirus covid
Read more »
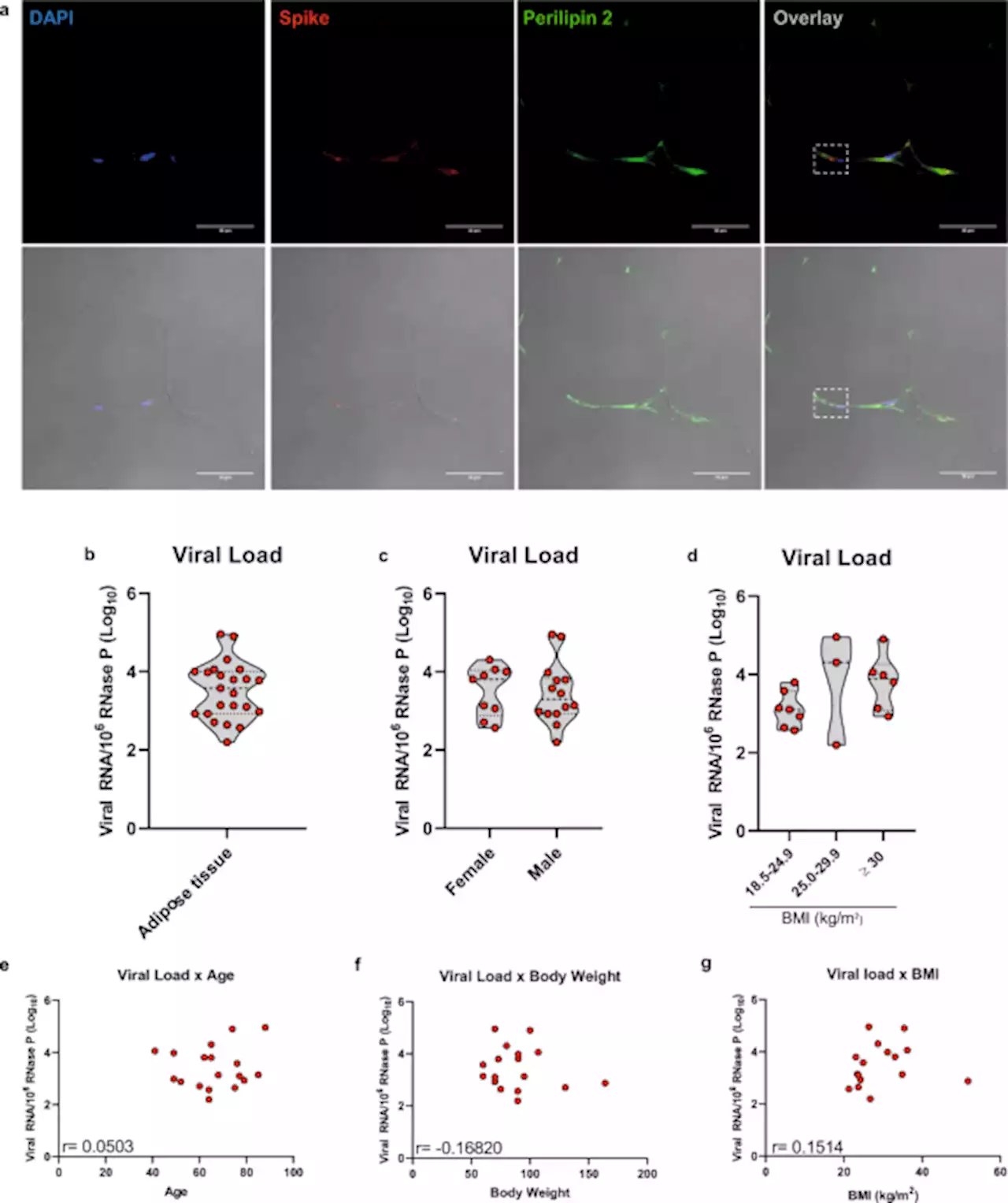 SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner - Nature CommunicationsVisceral adiposity is a risk factor for severe COVID-19, and infection of adipose tissue by SARS-CoV-2 has been reported. Here the authors confirm that human adipose tissue is a possible site for SARS-CoV-2 infection, but the degree of adipose tissue infection and the way adipocytes respond to the virus depend on the adipose tissue depot and the viral strain.
SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner - Nature CommunicationsVisceral adiposity is a risk factor for severe COVID-19, and infection of adipose tissue by SARS-CoV-2 has been reported. Here the authors confirm that human adipose tissue is a possible site for SARS-CoV-2 infection, but the degree of adipose tissue infection and the way adipocytes respond to the virus depend on the adipose tissue depot and the viral strain.
Read more »
 Machine learning analysis suggests that there are four sub-phenotypes of long COVIDMachine learning analysis suggests that there are four sub-phenotypes of long COVID NatureMedicine WeillCornell longCOVID coronavirus covid machinelearning data healthcaredata phenotype
Machine learning analysis suggests that there are four sub-phenotypes of long COVIDMachine learning analysis suggests that there are four sub-phenotypes of long COVID NatureMedicine WeillCornell longCOVID coronavirus covid machinelearning data healthcaredata phenotype
Read more »
 Is remdesivir treatment associated with reduced risk of inpatient mortality among US patients hospitalized with COVID-19?Researchers assessed the effect of remdesivir on inpatient COVID-19-associated mortality among individuals hospitalized with SARS-CoV-2 infections in an enormous dataset of US residents beyond clinical trial settings.
Is remdesivir treatment associated with reduced risk of inpatient mortality among US patients hospitalized with COVID-19?Researchers assessed the effect of remdesivir on inpatient COVID-19-associated mortality among individuals hospitalized with SARS-CoV-2 infections in an enormous dataset of US residents beyond clinical trial settings.
Read more »
 Interim analysis from a phase 2 randomized trial of EuCorVac-19: a recombinant protein SARS-CoV-2 RBD nanoliposome vaccine - BMC MedicineBackground Numerous vaccine strategies are being advanced to control SARS-CoV-2, the cause of the COVID-19 pandemic. EuCorVac-19 (ECV19) is a recombinant protein nanoparticle vaccine that displays the SARS-CoV-2 receptor-binding domain (RBD) on immunogenic nanoliposomes. Methods Initial study of a phase 2 randomized, observer-blind, placebo-controlled trial to assess the immunogenicity, safety, and tolerance of ECV19 was carried out between July and October 2021. Two hundred twenty-nine participants were enrolled at 5 hospital sites in South Korea. Healthy adults aged 19–75 without prior known exposure to COVID-19 were vaccinated intramuscularly on day 0 and day 21. Of the participants who received two vaccine doses according to protocol, 100 received high-dose ECV19 (20 μg RBD), 96 received low-dose ECV19 (10 μg RBD), and 27 received placebo. Local and systemic adverse events were monitored. Serum was assessed on days 0, 21, and 42 for immunogenicity analysis by ELISA and neutralizing antibody response by focus reduction neutralization test (FRNT). Results Low-grade injection site tenderness and pain were observed in most participants. Solicited systemic adverse events were less frequent, and mostly involved low-grade fatigue/malaise, myalgia, and headache. No clinical laboratory abnormalities were observed. Adverse events did not increase with the second injection and no serious adverse events were solicited by ECV19. On day 42, Spike IgG geometric mean ELISA titers were 0.8, 211, and 590 Spike binding antibody units (BAU/mL) for placebo, low-dose and high-dose ECV19, respectively (p | 0.001 between groups). Neutralizing antibodies levels of the low-dose and high-dose ECV19 groups had FRNT50 geometric mean values of 129 and 316, respectively. Boosting responses and dose responses were observed. Antibodies against the RBD correlated with antibodies against the Spike and with virus neutralization. Conclusions ECV19 was generally well-tolerated and induced antibodies
Interim analysis from a phase 2 randomized trial of EuCorVac-19: a recombinant protein SARS-CoV-2 RBD nanoliposome vaccine - BMC MedicineBackground Numerous vaccine strategies are being advanced to control SARS-CoV-2, the cause of the COVID-19 pandemic. EuCorVac-19 (ECV19) is a recombinant protein nanoparticle vaccine that displays the SARS-CoV-2 receptor-binding domain (RBD) on immunogenic nanoliposomes. Methods Initial study of a phase 2 randomized, observer-blind, placebo-controlled trial to assess the immunogenicity, safety, and tolerance of ECV19 was carried out between July and October 2021. Two hundred twenty-nine participants were enrolled at 5 hospital sites in South Korea. Healthy adults aged 19–75 without prior known exposure to COVID-19 were vaccinated intramuscularly on day 0 and day 21. Of the participants who received two vaccine doses according to protocol, 100 received high-dose ECV19 (20 μg RBD), 96 received low-dose ECV19 (10 μg RBD), and 27 received placebo. Local and systemic adverse events were monitored. Serum was assessed on days 0, 21, and 42 for immunogenicity analysis by ELISA and neutralizing antibody response by focus reduction neutralization test (FRNT). Results Low-grade injection site tenderness and pain were observed in most participants. Solicited systemic adverse events were less frequent, and mostly involved low-grade fatigue/malaise, myalgia, and headache. No clinical laboratory abnormalities were observed. Adverse events did not increase with the second injection and no serious adverse events were solicited by ECV19. On day 42, Spike IgG geometric mean ELISA titers were 0.8, 211, and 590 Spike binding antibody units (BAU/mL) for placebo, low-dose and high-dose ECV19, respectively (p | 0.001 between groups). Neutralizing antibodies levels of the low-dose and high-dose ECV19 groups had FRNT50 geometric mean values of 129 and 316, respectively. Boosting responses and dose responses were observed. Antibodies against the RBD correlated with antibodies against the Spike and with virus neutralization. Conclusions ECV19 was generally well-tolerated and induced antibodies
Read more »
