The FDA has approved daily disposables that release anti-allergy medication. Experts hope lenses could one day help treat cataracts and glaucoma.
upon us, and for the 40 percent of contact lens wearers who suffer from itchy eyes, there’s a new option for how to treat them: the first lens that can deliver a drug directly to the eye. Made by Johnson & Johnson, they contain the antihistamine ketotifen and were approved by the US Food and Drug Administration earlier this month.
Drug-eluting contact lenses could not only be more comfortable but also deliver more medication into the eye than drops do. However, it’s been a challenge to marry a drug with a specific contact lens. Soft contacts are made of a flexible, water-absorbing plastic called hydrogel that allows oxygen to pass through. But their properties vary from brand to brand.
J&J’s contacts are daily disposables, meaning they’re worn for a day and thrown out. In Phase 3 clinical studies, the contacts reduced itchiness from allergies as quickly as three minutes after putting the lenses in, and the effect lasted up to 12 hours. “When the lens is put on the eye, you get a very rapid release of medication to the eye, and then as the medication continues to elude out over the next several hours, it's a much slower release,” says Pall.
Ireland Latest News, Ireland Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 As FDA OKs Another COVID Booster, Some Experts Question NeedThe FDA today authorized Americans over the age of 50 to receive a second COVID-19 booster shot, even though many top infectious disease experts questioned the need before the agency’s decision.
As FDA OKs Another COVID Booster, Some Experts Question NeedThe FDA today authorized Americans over the age of 50 to receive a second COVID-19 booster shot, even though many top infectious disease experts questioned the need before the agency’s decision.
Read more »
 The FDA is expected to authorize 2nd boosters for people 50 and upAnyone 50 years and older could soon be eligible for a second booster dose of the Moderna or Pfizer COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
The FDA is expected to authorize 2nd boosters for people 50 and upAnyone 50 years and older could soon be eligible for a second booster dose of the Moderna or Pfizer COVID-19 vaccine. The plan comes as evidence increases that protection from three shots is fading and a fourth shot would help boost immunity back up.
Read more »
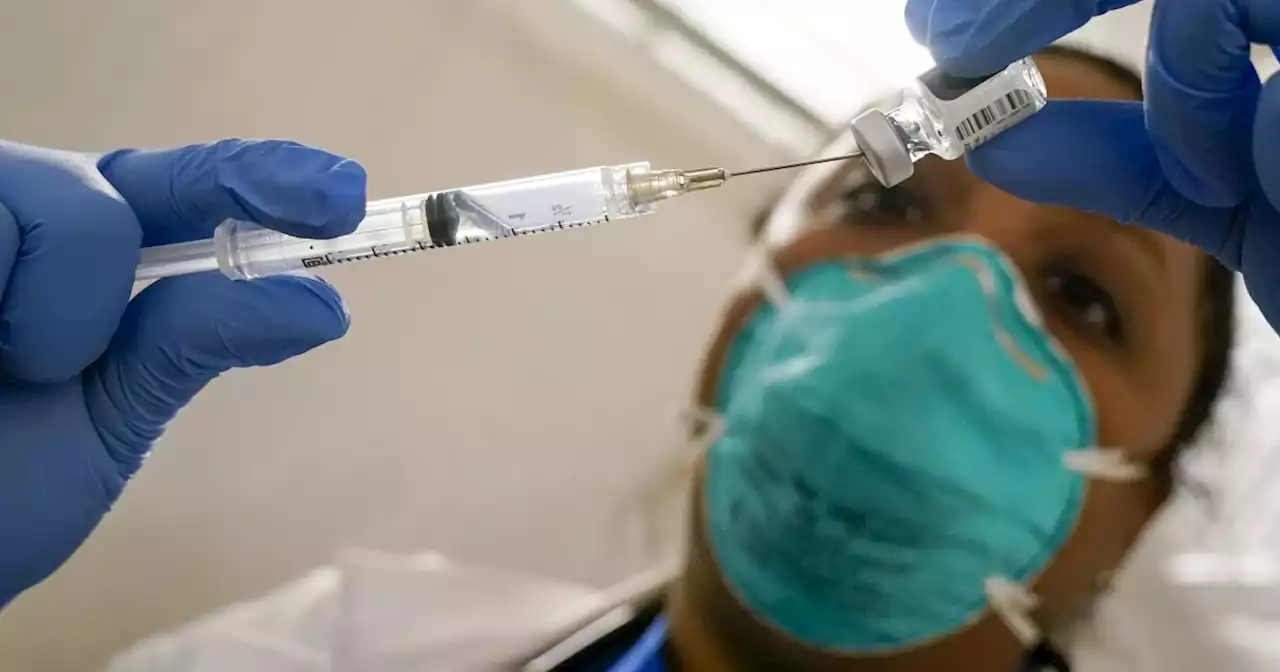 The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
Read more »
 FDA skeptical of benefits from experimental ALS drugThe drug from Amylyx Pharmaceuticals has become a rallying cause for patients with the deadly neurodegenerative disease ALS, their families and members of Congress who’ve joined in pushing the FDA to greenlight the drug.
FDA skeptical of benefits from experimental ALS drugThe drug from Amylyx Pharmaceuticals has become a rallying cause for patients with the deadly neurodegenerative disease ALS, their families and members of Congress who’ve joined in pushing the FDA to greenlight the drug.
Read more »
 FDA skeptical of benefits from experimental ALS drugThe Food and Drug Administration has issued a negative review of a closely watched experimental drug for the debilitating illness known as Lou Gehrig’s disease
FDA skeptical of benefits from experimental ALS drugThe Food and Drug Administration has issued a negative review of a closely watched experimental drug for the debilitating illness known as Lou Gehrig’s disease
Read more »
 FDA Skeptical of Benefits From Experimental ALS DrugThe Food and Drug Administration has issued a negative review of a closely watched experimental drug for the debilitating illness known as Lou Gehrig’s disease
FDA Skeptical of Benefits From Experimental ALS DrugThe Food and Drug Administration has issued a negative review of a closely watched experimental drug for the debilitating illness known as Lou Gehrig’s disease
Read more »
